The 21 CFR Part 11 module is available on Enterprise plans.
Our 21 CFR Part 11 module enables organisations that operate under FDA regulations to maintain and meet FDA compliance.
Video Tutorial
Watch our video tutorial or follow the steps below.
Step 1
From the dashboard, go to ‘Get Started’ or ‘Templates’.
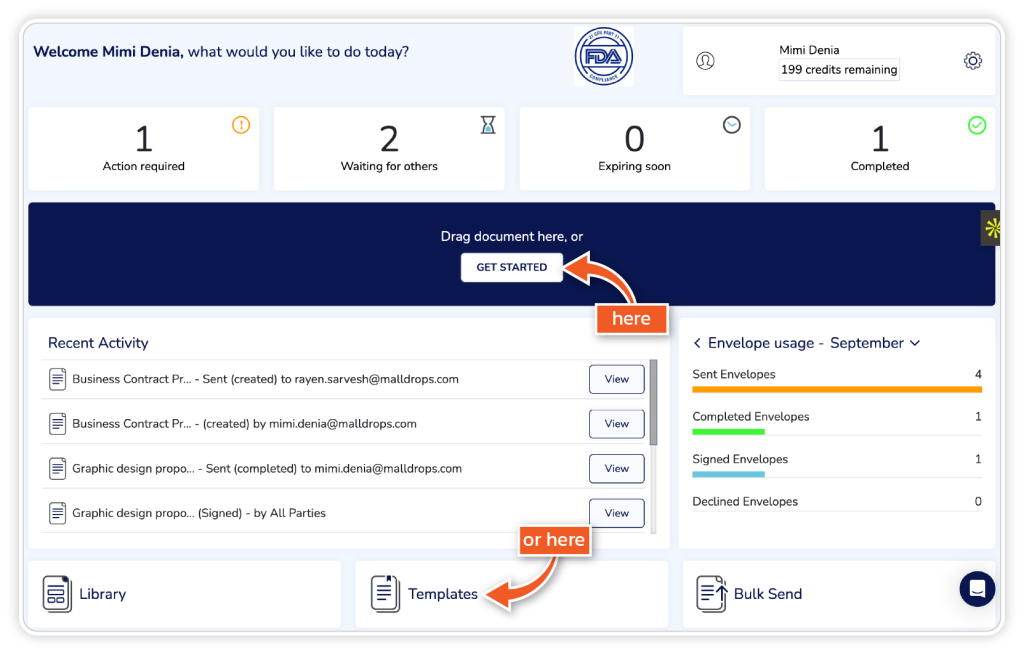
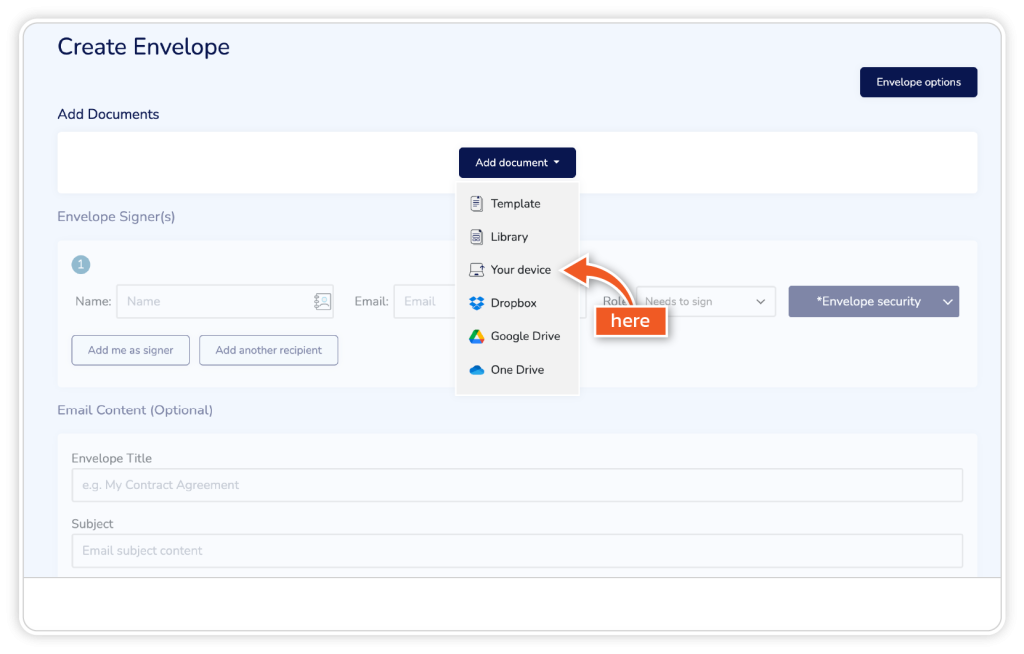
Step 2
Add your document through one of the listed options.
Step 3
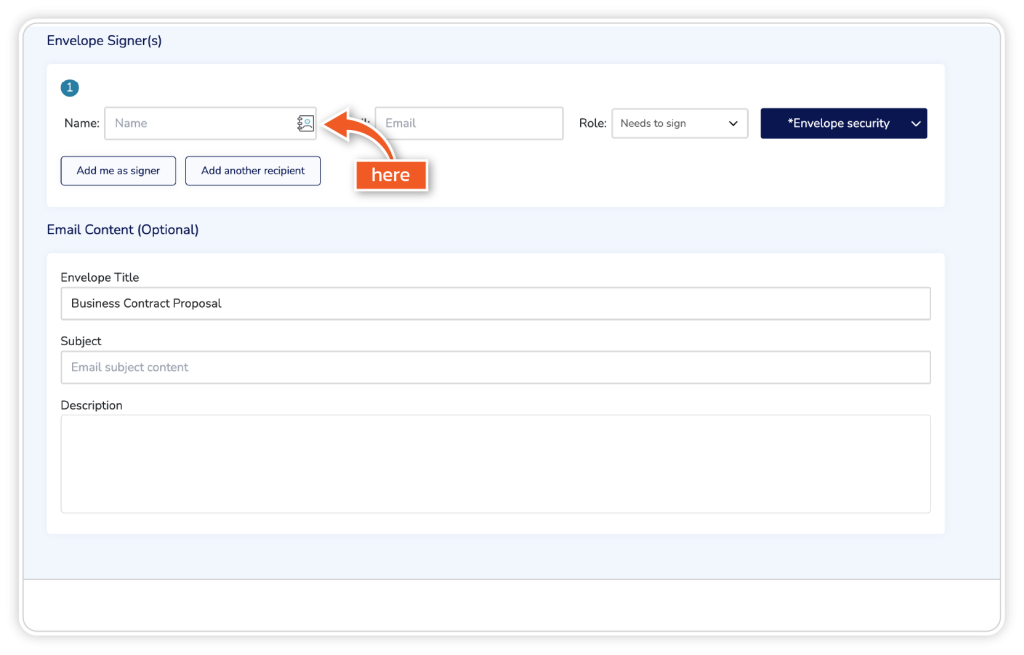
Under Envelope Signers, add the signer’s full name and email address. Alternatively, you can access signer details via the address book symbol.
Step 4
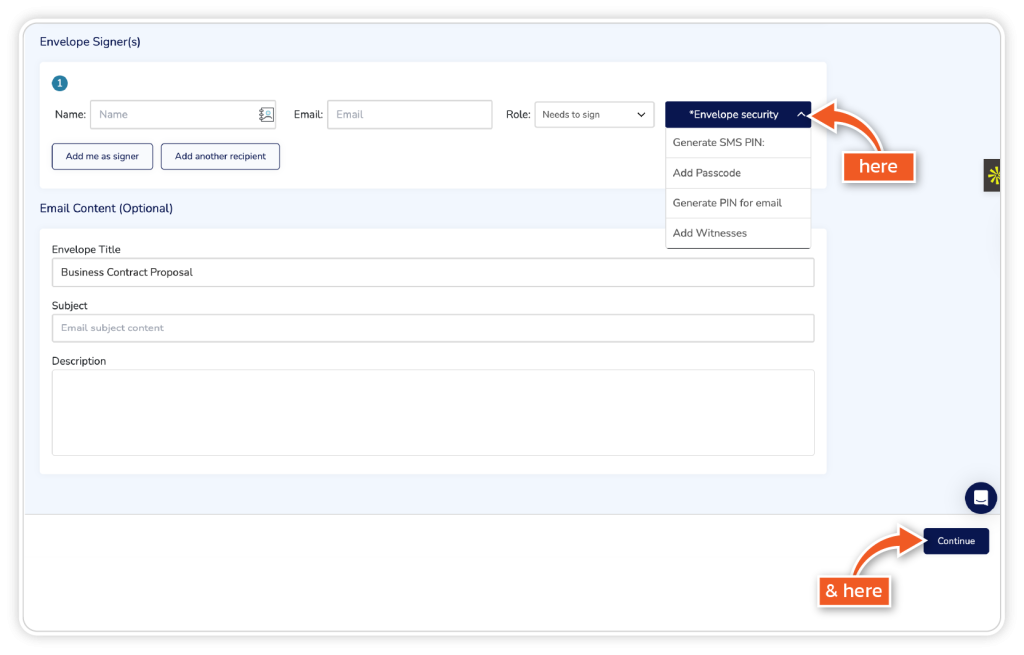
Click ‘Envelope Security’ and choose one of the options in the dropdown for each signer individually. Then, complete the email section if applicable and click ‘Continue’.
Step 5
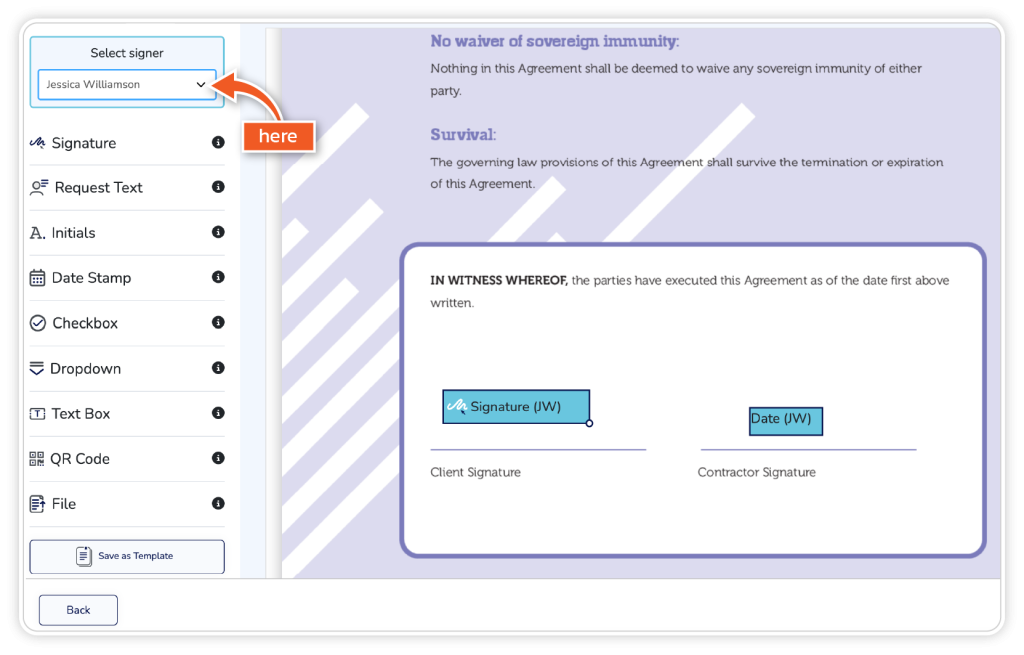
Drag and drop your required fields onto the document. If you have multiple signers, switch between signers in the top left when inputting fields.
Step 6
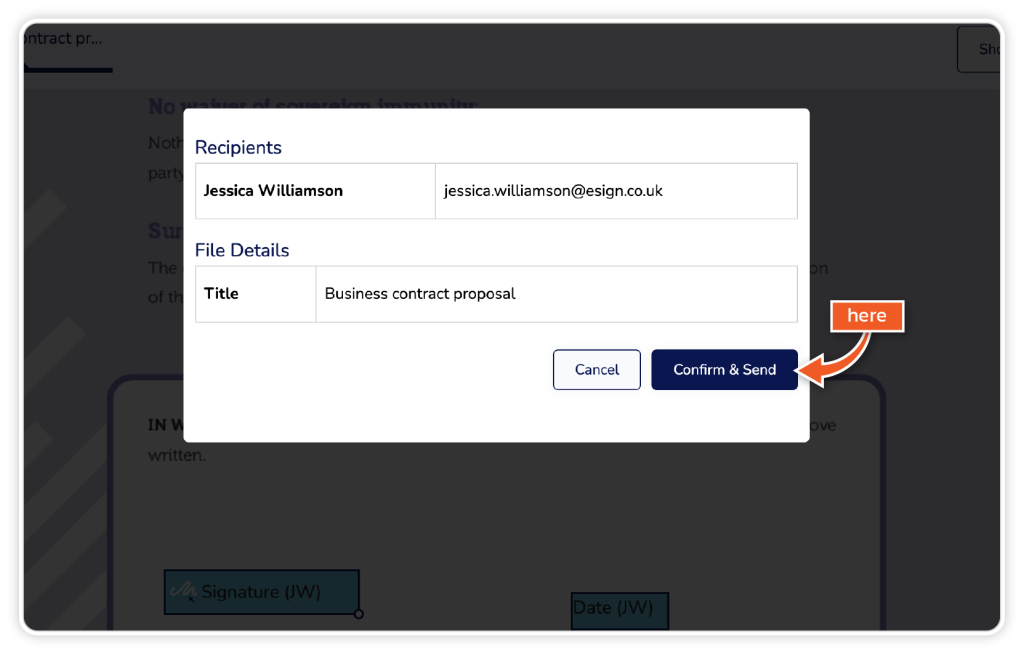
Click ‘Continue’ when all the relevant fields are added, and then click ‘Confirm and Send’.